Crystagen 20mg
$65.00
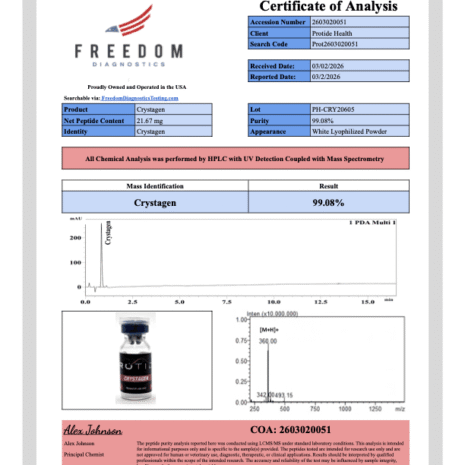
Crystagen (Glu-Asp-Pro tripeptide) investigated in preclinical T-cell differentiation, cellular homeostasis, and epigenetic signaling models. Supplied as a stable 20mg lyophilized vial. Third-party HPLC-verified >99% purity. For research use only.
In stock
Free shipping on orders over $149!
- 99% Purity (HPLC-MS Verified)
- Independent Third-Party Tested
- USA GMP Manufactured
- Complimentary BAC Water
- Ships in 24 Hours (USA)
Disclaimer: This product is intended solely for laboratory research purposes. It is not for human consumption, medical use, veterinary use, or household application. All product information on this website is provided for educational purposes only. Researchers must handle this product with appropriate safety protocols and comply with all applicable regulations. Please review our Terms & Conditions before purchasing.
Crystagen (EDP) 20mg Peptide
Crystagen is an ultrashort synthetic tripeptide sequence (Glu-Asp-Pro or EDP) engineered to mirror the biologically active components found in naturally occurring thymic extracts. In modern laboratory settings, Crystagen is heavily investigated as a bioregulatory compound, providing researchers with a highly precise molecular tool for studying cellular immune homeostasis, epigenetic signaling, and T-cell marker modulation.
For investigators looking to buy Crystagen peptide for their in vitro or animal models, Protide Health supplies this ultrashort sequence as a stable, lyophilized powder. By offering a standardized 20mg formulation, researchers can ensure consistent dose-dependent modeling across complex cellular proliferation and chromatin state assays.
Peptide Type: Ultrashort Regulatory Tripeptide
Vial Content: 20mg Crystagen (Glu-Asp-Pro)
Form: Lyophilized (Freeze-Dried) for maximum biochemical stability
Mechanism of Action: Epigenetic Signaling and Immune Modulation
Crystagen is synthesized to investigate the core pathways of cellular gene regulation. Unlike larger peptides that primarily bind to extracellular receptors, ultrashort tripeptides like EDP are actively researched for their ability to penetrate the cell nucleus and directly interact with DNA and histone targets.
Gene-Regulatory and Chromatin Effects
In in vitro models, EDP is observed binding directly to target sites, modulating the transcription of genes related to heat-shock responses and cell-cycle control. In assays examining aged lymphocytes, Crystagen administration has been reported to promote heterochromatinization. Researchers utilize this mechanism to track shifts in chromatin states during modeled cellular senescence and immune aging.
Oligopeptide Transporter Assays
Due to its ultrashort molecular weight (~359.3 Da), Crystagen serves as a premier model for studying cellular uptake via proton-dependent oligopeptide transporters (PEPT1/PEPT2) and LAT1/2 carriers. This allows laboratories to map tissue-specific absorption pathways without the interference common to larger, more complex molecular structures.
Advanced Research Applications for Crystagen
1. T-Cell Differentiation & Cytokine Expression
Researchers seeking Crystagen 20mg for sale frequently utilize it in flow-cytometry panels to study immune cell programming. In vitro assays track how EDP influences the differentiation and proliferation of T-helper cells and thymocytes, specifically observing shifts in CD3+ and CD4+ markers and the normalization of CD4+/CD8+ ratios in isolated cellular environments.
2. Cellular Quality-Control & Heat-Shock Pathways
Crystagen is heavily researched in models of high physiological cellular stress. Investigators track the upregulation of heat-shock proteins (specifically HSPA1A or HSP70) and the modulation of IL-6 expression to understand how tripeptides influence cellular resilience and cytokine gene expression during accelerated stress modeling (such as γ-irradiation assays).
3. Macrophage & Epithelial Cell Function
Preclinical laboratory studies utilize Crystagen to observe selective proliferation effects. Research indicates that EDP can stimulate the proliferation of normal human lymphocytes while inhibiting specific immortalized or tumor cell lines. Furthermore, it is used to activate human thymic epithelial cells and map cytokine secretion (IL-1, TNF-α) from isolated murine macrophages.
Specifications Table: Crystagen (EDP)
| Parameter | Technical Details |
|---|---|
| Product Name | Crystagen (Glu-Asp-Pro) |
| Sequence | EDP (Glutamic Acid – Aspartic Acid – Proline) |
| Vial Weight | 20mg (Active Peptide) |
| Molecular Weight | ~359.3 Da |
| Purity Grade | 99%+ (Verified via HPLC/MS) |
| Solubility | Reconstitute with Bacteriostatic Water |
| Intended Use | Laboratory Research Only |
Where is the best place to buy Crystagen online for research?
Qualified laboratories searching to buy Crystagen online can acquire it directly through Protide Health. We provide high-yield Crystagen 20mg vials manufactured exclusively for in vitro and preclinical animal modeling. Every batch undergoes strict third-party HPLC testing to ensure structural integrity and >99% purity.
Why do researchers prefer the 20mg vial size for Crystagen?
Ultrashort regulatory peptides often require a wide range of concentration testing to establish accurate dose-response curves in gene-expression panels. The Crystagen 20mg size provides ample compound for extended, multi-phase in vitro assays (such as qPCR panels or flow-cytometry), minimizing the variable of batch-to-batch inconsistency.
How should Crystagen be stored in the laboratory?
To maintain the stability of the ultrashort EDP sequence, sealed vials must be stored in a cool, dry, and dark environment (typically -20°C for long-term storage). Once reconstituted with bacteriostatic water, the solution must be handled according to strict institutional SOPs and refrigerated to prevent degradation.
Conclusion: Summary for Researchers
Crystagen (EDP) is a highly defined ultrashort regulatory tripeptide offering profound potential as a research tool. Characterized by its ability to enter the cell nucleus and engage directly with DNA and histones, it provides a unique mechanistic rationale for studying gene expression, immune profile normalization, and cellular stress responses. Available exclusively for laboratory use, the 20mg Crystagen formulation is an indispensable asset for advancing preclinical immunology and epigenetic discovery.
Citations
Khavinson V et al., 2021. The Use of Thymalin for Immunocorrection and Molecular Aspects of Biological Activity. International Journal of Molecular Sciences.
Fedoreyeva LI et al., 2011. Penetration of short peptides into the nucleus and their interaction with DNA. Biochemistry (Moscow).
Khavinson V et al., 2022. Peptide Regulation of Gene Expression: A Systematic Review. Molecules.
Khavinson V et al., 2012. Effect of ultrashort peptides on proliferation of normal and tumor cells. Bulletin of Experimental Biology and Medicine.
Legal Disclaimer
Crystagen (EDP) is intended for laboratory research purposes only. It is not approved by the U.S. Food and Drug Administration (FDA) or any regulatory authority for human consumption, medical use, or therapeutic application. It is intended solely for investigational use in controlled laboratory settings by qualified researchers. Protide Health does not endorse or promote the use of Crystagen in humans or animals outside of approved research protocols. Researchers must comply with all applicable local, state, and federal regulations, including obtaining necessary approvals for experimental use.