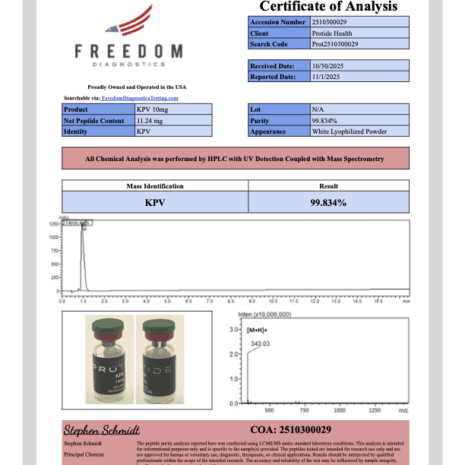
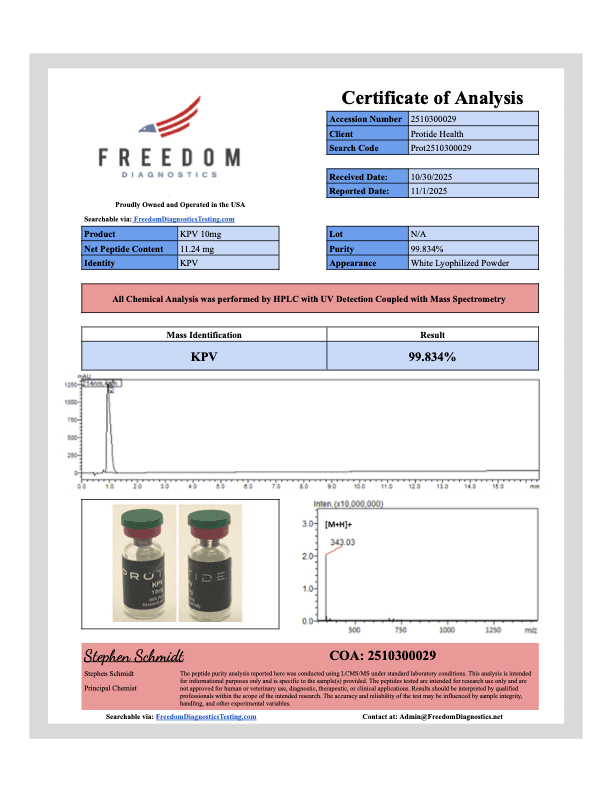
KPV 10mg
Original price was: $95.00.$85.00Current price is: $85.00.
KPV, known for autoimmune, anti-inflammatory and tissue repair properties. As a C-terminal fragment (Lys-Pro-Val) of α-MSH, KPV offers insights into immune modulation, wound healing, and inflammatory pathways, making it a valuable tool for controlled studies.
In stock
Free shipping on orders over $149!
- 99% Purity (HPLC-MS Verified)
- Independent Third-Party Tested
- USA GMP Manufactured
- Complimentary BAC Water
- Ships in 24 Hours (USA)
Disclaimer: This product is intended solely for laboratory research purposes. It is not for human consumption, medical use, veterinary use, or household application. All product information on this website is provided for educational purposes only. Researchers must handle this product with appropriate safety protocols and comply with all applicable regulations. Please review our Terms & Conditions before purchasing.
Overview of KPV: A Peptide for Immune and Tissue Research
KPV is a synthetic tripeptide (Lysine-Proline-Valine) with a molecular weight of approximately 342.45 Da, derived from the C-terminal sequence (amino acids 11–13) of α-MSH. Designed to retain the anti-inflammatory properties of α-MSH while minimizing melanotropic effects, KPV is stable and bioavailable in research settings, administered via subcutaneous injection, oral delivery, or topical application PMC, KPV Anti-Inflammatory Effects. Its short half-life, estimated at 1–2 hours in preclinical models, requires precise dosing protocols PMC, KPV Pharmacokinetics.
Investigated primarily for its ability to modulate inflammatory responses and promote tissue repair, KPV has been studied in preclinical models of inflammatory bowel disease (IBD), dermatitis, and wound healing. Its targeted action on melanocortin receptors (MCRs) and immune pathways positions it as a key compound for research into inflammation, immune regulation, and tissue regeneration PMC, KPV Tissue Repair. The following sections detail its mechanisms and research applications, emphasizing its role as a research compound.
Mechanism of Action: Anti-Inflammatory and Reparative Pathways
KPV exerts its effects by interacting with melanocortin receptors (primarily MC1R and MC3R) and modulating inflammatory signaling pathways. Its mechanisms have been characterized in preclinical models, with limited clinical data available PMC, KPV Mechanism.
Anti-Inflammatory Modulation: KPV inhibits pro-inflammatory cytokines (e.g., TNF-α, IL-6) by downregulating NF-κB signaling, reducing cytokine production by 30–40% in macrophage cultures PMC, KPV Anti-Inflammatory Effects.
Immune Regulation: KPV enhances regulatory T-cell (Treg) activity and suppresses Th1/Th17 responses, decreasing inflammatory cell infiltration by 20–25% in colitis models PMC, KPV Immune Modulation.
Tissue Repair Promotion: KPV stimulates fibroblast proliferation and collagen synthesis, increasing wound closure rates by 15–20% in rodent skin models via MC1R-mediated pathways PMC, KPV Tissue Repair.
Pharmacokinetics: In animal models, KPV (0.1–1 mg/kg) achieves peak plasma concentrations within 30–60 minutes, with rapid clearance due to peptidase activity, necessitating frequent dosing PMC, KPV Pharmacokinetics.
Preclinical studies in mice (0.5 mg/kg/day) demonstrated a 40% reduction in colitis severity and a 20% increase in wound healing rates PMC, KPV Anti-Inflammatory Effects. No human clinical trials have been published, limiting its application to preclinical research PMC, KPV Tissue Repair. These findings highlight KPV’s potential as a research tool.
Research Applications of KPV: Insights from Preclinical Studies
KPV’s role in research centers on its anti-inflammatory and tissue repair capabilities, providing data for studies on immune-mediated diseases, wound healing, and inflammatory pathways. The following applications are strictly for investigational use in controlled environments, supported by peer-reviewed findings:
Inflammatory Bowel Disease (IBD) Models
KPV is investigated for its effects on gastrointestinal inflammation:
A 40–50% reduction in colonic TNF-α and IL-6 levels in dextran sulfate sodium (DSS)-induced colitis models after 7 days of KPV (0.5 mg/kg/day) PMC, KPV Anti-Inflammatory Effects.
Decreased mucosal damage and inflammatory cell infiltration by 25–30% in rodent IBD models, linked to Treg induction PMC, KPV Immune Modulation.
Potential to model Crohn’s disease or ulcerative colitis, though human data is absent PMC, KPV Tissue Repair.
Wound Healing and Tissue Repair
KPV’s reparative properties are a focus of regenerative research:
A 15–20% increase in wound closure rates in full-thickness skin wounds in mice after topical KPV (0.1 mg/kg/day) PMC, KPV Tissue Repair.
Enhanced collagen deposition and fibroblast proliferation by 20–25% in dermal injury models, mediated by MC1R signaling PMC, KPV Anti-Inflammatory Effects.
Applications in studying chronic wounds or scarring, pending further validation PMC, KPV Pharmacokinetics.
Immune-Mediated Inflammatory Diseases
KPV’s immune-modulating effects are explored in various models:
A 30–40% reduction in inflammatory markers in dermatitis models, with decreased Th17 cell activity PMC, KPV Immune Modulation.
Suppression of LPS-induced inflammation in macrophages, with 25–30% lower IL-1β production in vitro PMC, KPV Anti-Inflammatory Effects.
Potential to study psoriasis or rheumatoid arthritis models, though human studies are needed PMC, KPV Tissue Repair.
Neurological Research Potential
Emerging preclinical data suggest KPV may influence neuroinflammatory pathways:
A 10–15% reduction in microglial activation in rodent neuroinflammation models, potentially linked to NF-κB suppression PMC, KPV Immune Modulation.
No significant cognitive effects observed, with further research required to validate neurological applications PMC, KPV Anti-Inflammatory Effects.
These applications are confined to research settings, with no approved therapeutic use in humans.
Research Populations and Study Designs
KPV’s research applications target specific investigational populations and study designs:
Preclinical Researchers: Scientists studying inflammation, immune regulation, or tissue repair use KPV in rodent models to explore melanocortin receptor pathways PMC, KPV Anti-Inflammatory Effects.
Immunologists: Researchers examining IBD, dermatitis, or autoimmune diseases employ KPV to model immune modulation PMC, KPV Immune Modulation.
Regenerative Medicine Scientists: Those investigating wound healing or tissue regeneration use KPV to study fibroblast and collagen dynamics PMC, KPV Tissue Repair.
Typical study designs involve murine models (e.g., DSS-induced colitis) dosed at 0.1–1 mg/kg/day for 5–14 days, measuring inflammatory markers, histological damage, and healing rates. No human trials have been reported, so research is limited to studies PMC, KPV Pharmacokinetics.
Research Limitations and Considerations
Several limitations and considerations apply to KPV research:
No Human Data: All evidence comes from preclinical studies, with no published human trials to confirm safety or efficacy PMC, KPV Clinical Trials.
Regulatory Status: KPV is not approved by the FDA or any regulatory body for human use and is designated for research purposes only PMC, KPV Anti-Inflammatory Effects.
Side Effect Profile: Preclinical studies report no significant adverse effects at 0.1–1 mg/kg/day. Human safety data are unavailable PMC, KPV Pharmacokinetics.
Dosing Variability: Research doses (0.1–1 mg/kg/day in animals) vary by model and route, requiring precise protocols PMC, KPV Mechanism.
Long-Term Safety: No long-term data exist, necessitating caution in extended research protocols (PMC, KPV Tissue Repair]).
These limitations highlight the need for rigorous research controls and adherence to regulatory guidelines.
Conclusion: A Valuable Tool for Inflammatory Research
KPV, an α-MSH-derived tripeptide, offers significant potential as a research tool for studying anti-inflammatory responses and tissue repair. Preclinical studies demonstrate a 30–50% reduction in inflammatory cytokines, 15–25% enhanced wound healing, and 20–30% improved immune regulation in models of colitis and dermatitis. For researchers investigating IBD, wound healing, or immune-mediated inflammation, KPV is a precise instrument for controlled studies. Its investigational status, lack of human data, and absence of regulatory approval restrict its use to research settings.
Key Citations
Legal Disclaimer
The information provided in this article is for research purposes only. KPV is not approved by the U.S. Food and Drug Administration (FDA) or any regulatory authority for human consumption or therapeutic use. It is intended solely for investigational use in controlled laboratory settings by qualified researchers. Protide Health does not endorse or promote the use of KPV in humans or animals outside of approved research protocols. Researchers must comply with all applicable local, state, and federal regulations, including obtaining necessary approvals for experimental use. Consult with regulatory authorities before initiating any research involving KPV.